Mercury adsorption and reduction by nonlinear optical material (NLOM) DMABR loaded on Sepiolite: A mechanism study
Fig. Graphical abstract
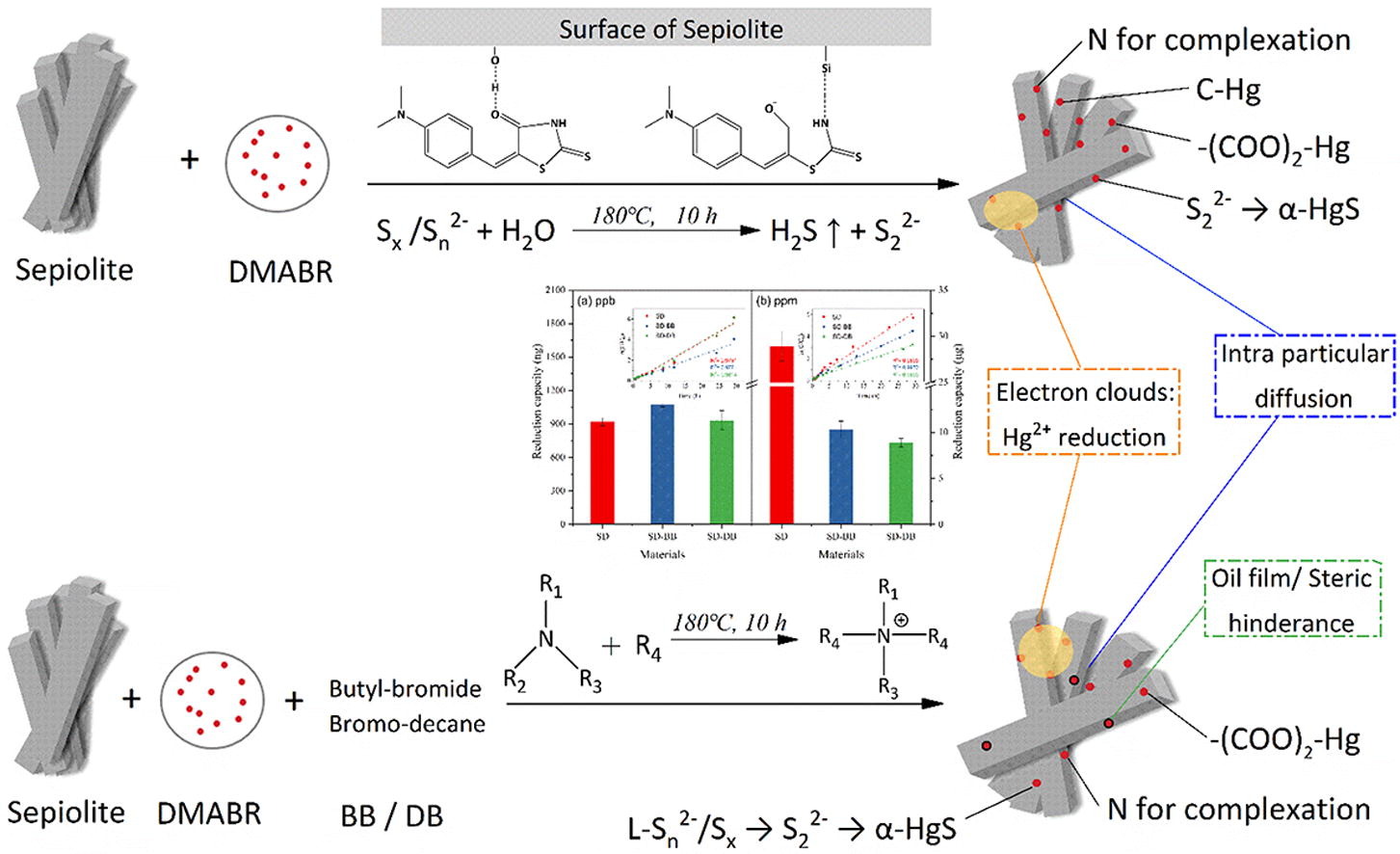
Adsorption and reduction techniques are the main approaches for Hg2+ remediation. The delocalized electrons of nonlinear optical materials (NLOM) can be used as electron donors, while reduction remediation material based on this has not yet been reported. In this work, nonlinear optical materials (NLOM) DMABR loaded on sepiolite (SD material) was successfully synthesized via one-step hydrothermal treatment. The results of FTIR, SEM and UV–vis spectra suggested that the load material (SD) was well functionalized. Removal (adsorption and reduction) kinetics illustrated remediation potential of SD for Hg2+. Hg2+ can be effectively removed by the developed materials, with a capacity up to 68.5 mg/g. The high tolerance capacity of SD against interfering ions and organic species makes it appropriate for selective and efficient removal of Hg2+ from aqueous solutions. Characterized by XRD, Raman spectroscopy, XPS spectra, Zeta potential and elemental analyzer, the removal mechanism was elucidated. Under mercury stress, Sn2- in SD was decomposed into S22- and the coordination number with N increased, resulting in formation of α-HgS and inversion asymmetric products. It was proved that the reduction sites were at the strong chelate groups (N and S) due to the high content of oxidized states in spent samples. By adding mercury stepwise, the kinetics mechanism could be readily inferred by the changing emission intensity at 564 nm, providing a simpler way to study the kinetics of adsorption of contaminants using fluorescent materials. Taken together, our work showed that NLOM can participate in the redox electron transport chain of pollutants such as mercury, and thus offers promising potential for application in remediation of mercury contamination.
Source: Chemical Engineering Journal