
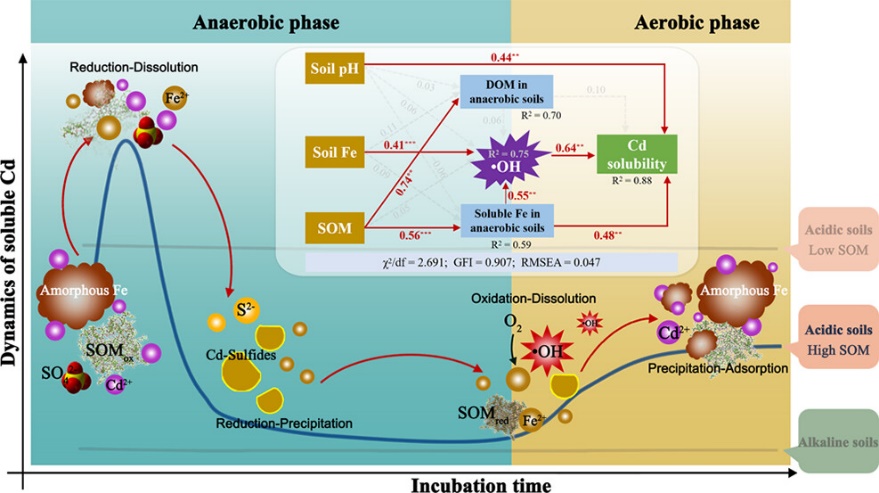
Soil flooding decreases the solubility of cadmium (Cd) by forming Cd-sulfides. However, hydroxyl radicals (•OH) can induce the oxidative dissolution of Cd-sulfides, increasing Cd solubility. The mechanisms underlying •OH generation and its subsequent effects on Cd solubility during soil redox fluctuations remain unclear. This study investigated the dynamics of soluble Cd during a 40-day anaerobic incubation followed by a 15-day aerobic incubation of Cd-contaminated soils (3.73–4.48 mg/kg). Prof. WANG Yujun and his team focused on the yields and generation mechanisms of •OH during oxygenation. Significant redox reactions involving reduced Fe, Mn, and soil organic matter (SOM) occurred in soils with high SOM and amorphous Fe content. In contrast, minimal changes in the concentrations of soluble metals were observed in soils with low content of SOM or amorphous Fe. During the anaerobic phase, biogenic S2– precipitated with soluble Cd to form Cd-sulfides. The oxygenation of reduced Fe and SOM during the subsequent aerobic phase led to •OH generation (0.20–0.56 mmol/kg soil), which induced the oxidative dissolution of Cd-sulfides and increased Cd solubility in soils. Their findings indicate that •OH generation driven by redox fluctuations enhances Cd solubility, emphasizing the critical role of •OH in modulating the migration and bioavailability of Cd in soils.
This study published in ACS ES&T Water in March 2025.
Graphical abstract
Attachment Download: