The heavy use of veterinary antibiotics in intensive animal husbandry and aquiculture, for treatment of infectious diseases and especially as animal feed additives has led to serious environmental problems such as the emergence of drug resistance among pathogenic microbes, and even the formation of cross- and multiple- resistances in organisms. Therefore, it is of great importance to fully understand the occurrence, fate, transport and transformation of antibiotics. Biochar derived from burning plant residues is a ubiquitous geosorbent that is long lasting in the natural environment and thus influences the environmental behavior of antibiotics. However, a knowledge gap exists on the interaction between antibiotics and biochar.
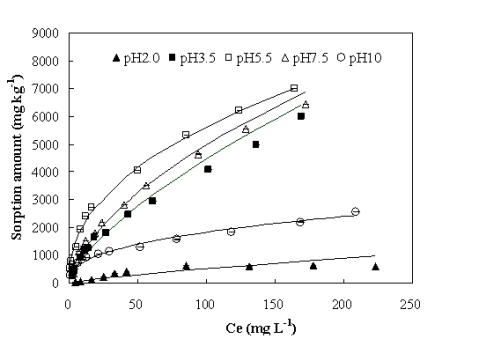
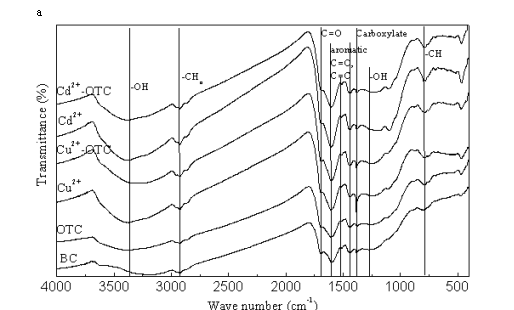
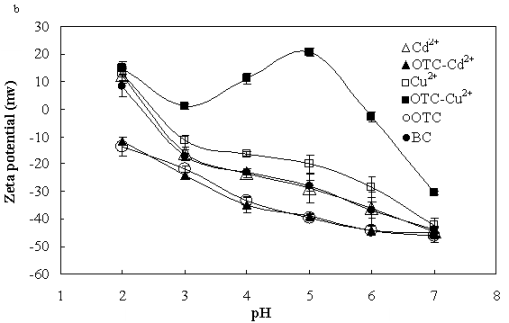
Dr. Xin Jiang’s group from the Institute of Soil Science, Chinese Academy of Sciences has found the main interaction mechanisms of antibiotics to biochar. They use oxytetracycline (OTC), one of the most widely used antibiotics in animal feeds and aquaculture in China as a model compound to reveal the sorption mechanisms of antibiotics to biochar: (1) OTC sorbed to biochar according to pseudo-second-order kinetics; (2) Sorption amount and sorption affinity of OTC to biochar were influenced by solution pH and the maximum sorption occurred at pH5.5 (Fig. 1); (3) The presence of heavy metals, some of which were usually used together with antibiotics as growth promoters, had different effects on OTC sorption to biochar: insignificant by Cd2+, slight facilitation by Zn2+, slight inhibition by Pb2+, and Cu2+ strongly enhanced the sorption of OTC to biochar through metal bridging (Fig.s 2, and 3); and (4) Zwitterions were the most sorbed oxytetracycline species on biochar and surface complexation, through π-π interaction as well as metal bridging, was the most important sorption mechanism although cation exchange might have played a role (Fig. 3).
These findings have been published on Bioresource Technology (Effects of pH and metal ions on oxytetracycline sorption to maize-straw-derived biochar, 136: 87-93). It has implication for using biochar from pyrolysis of plant residues to retain the ionizable antibiotics like tetracyclines even it was accompanied with heavy metals.
|
|
|
Fig. 1 Sorption isotherms of oxytetracycline (OTC) on biochar at different pH values.dots: measured data,solid lines: fitted curves with Freundlich equation at corresponding pH values.
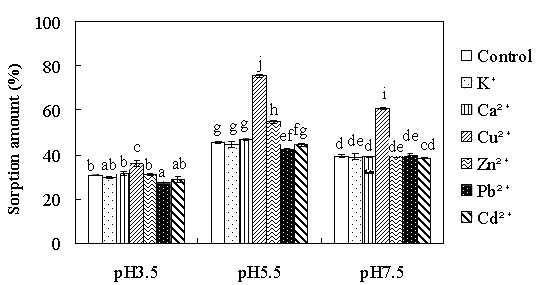
Fig. 2 Percentage of oxytetracycline (OTC) adsorbed on biochar at three pH values as affected by metal ions. The initial concentration of both OTC and metal ions are 0.2 mM. Statistically significant at a level of p<0.05 (Tukey HSD).
|
|
|
|
|
Fig. 3 FTIR spectra (a) and (b) Zeta potential of biochar (BC) before and after sorption of oxytetracycline (OTC), Cu2+, Cd2+, both OTC and Cu2+, and both OTC and Cd2+ |
|
.
|
